
Normally both the right and left atria i.e. the upper heart chambers are separated by an interatrial septum and the ventricles i.e. the lower heart chambers are separated by an interventricular septum. Any defect in these membranes is called septal defects i.e. ASD and VSD respectively. Also the main arteries arising from the heart i.e. the aorta and pulmonary arteries have a ductus arteriosus as a connection between them in intrauterine life (prior to birth) which normally closes soon after birth. Persistence of the same after birth is called PDA (patent ductus arteriosus).
To treat these defects, either a device is used or it is repaired with open heart surgery. These devices plug the hole in the membranes and seal off blood flow between the right and left chambers of the heart. Devices are made up of combinations of various metals like nitinol etc. They are mesh-like devices with a ring-like structure on each side so that it hugs the membranes on both ends to hold the device firmly in place.
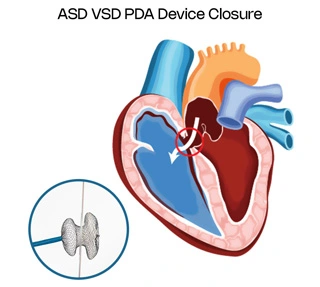
Defect in the membrane separating the right and left atria is called an atrial septal defect.
Sub Types of Atrial Septal Defect
PFO or patent foramen ovale - It is technically not a ASD. It is just that the septum primum and secundum fail to meet and close up. Normally it needs no treatment, but if patients have a stroke or recurrent migraine, then it may need to be closed.
Ostium Primum ASD - It occurs when the septum primum fails to fuse with the endocardial cushions and it is broadly associated with atrioventricular septal defects.
Ostium Secundum ASD - Most common type of ASD wherein the septum secundum fails to occlude the ostium.
Sinus Venosus ASD - These occur near the place in the septum where the superior or the inferior vena cava drain in the right atria. Coronary Sinus ASD - It occurs when there is unroofing of the coronary sinus, leading to blood flow from the left atria to right atria via the coronary sinus.
Symptoms of ASD
Many times they are asymptomatic in children and are diagnosed incidentally when Echocardiogram is done for other reasons.
In adults, they may present with tiredness, shortness of breath, palpitations or swelling over the legs. In late cases patients can have cyanosis i.e. bluish tinge of lips and ears and thickening and rounding of fingernails called clubbing.
Defect in the membrane separating the right and left ventricle is called a ventricular septal defect.
Subtypes of VSD
Membranous VSD - the defect is present in the upper part of the ventricular septum. It is the most common VSD and accounts for more than 75% of VSDs.
Muscular VSD - The defect is usually present in the mid to lower part of the ventricular septum. These account for around 20-25% of VSDs.
Outlet VSD - In the form of subaortic or subpulmonic VSDs. This defect is present in the septum just before the aortic or pulmonary arteries arise.
Inlet VSD - This defect is present in the septum located close to the tricuspid or the mitral valves.
Post Infarct VSD - This occurs secondary to extensive myocardial infarction or heart attack, that causes thinning and eventually rupture of the septum causing a secondary VSD due to loss of blood supply to that part of the septum. This is a serious and life threatening condition.
Symptoms and complications of untreated VSD
Symptoms of VSD depend on its location and size of defect. Very small VSD are asymptomatic and diagnosed when a physician hears a murmur or Echocardiogram is done for other reasons.
Bigger defects can cause symptoms of shortness of breath or tiredness, palpitations and swelling over the feet. The bigger the defect, the earlier the symptoms arise.
As mentioned previously, PDA is the persistence of connection between the aorta and the pulmonary artery, a condition leading to left to right shunting of blood at the heart level.
Symptoms and complications of untreated PDA.
It depend on its size, very small PDA are asymptomatic and diagnosed when a physician hears a murmur or Echocardiogram is done for other reasons. Bigger defects can cause symptoms of shortness of breath or tiredness, palpitations and swelling over the feet. The bigger the defect, the earlier the symptoms arise.
When the shunting of blood is significant i.e. the Qp:Qs is more than 2:1 (blood flowing in pulmonary artery to aorta ratio), device closure is needed. If the patients have symptoms and the ratio is less than 2:1, then also in some cases closure may be considered. Very rarely it may be considered to close even if the defect is small, to reduce the risk of infective endocarditis. This is mainly for small PDA and occasionally for VSD. Thus right patient selection and counselling of risk and benefits with them is extremely important.
Diagnostic
Pre Procedural Preparation
Preoperative assessments, blood tests, ECG, Echocardiogram is done prior to posting the patients for a procedure. Patients and their relatives are counselled in terms of risks and benefits and written informed consent is taken.
Specifics Steps of Device Closure
Post Procedural Care
Patients are monitored in the hospital overnight and groin site is also monitored. Next day an echocardiogram is done to confirm the positioning and if there are no issues, patients can go home the next day. Patients are ambulatory the next day and can resume all activities in a few weeks time.
Long-term Follow-up and Monitoring
Annual follow ups with your doctor is recommended for the first few years with an echocardiogram to monitor the device functioning and position. Care has to be taken for a few months post procedure to avoid direct trauma to the chest. Post that, patients can resume all their daily activities.
Surgical vs. Non-surgical Options
There is always a debate about treatment modality for defect repair, whether to go for surgical correction or to proceed with device closure. The crux of the matter is, if the defect is feasible for device closure, then we should opt for it as the first option. If not, then surgical correction will be the choice for the treatment.
Clinical Outcomes and Success Rates
Device closure rates are more than 90% if candidates are chosen with meticulous planning. Post the procedure, there will be significant improvement in the quality of life and in most cases, there will also be better longevity. Once the device is placed and has been stable for a few weeks, it remains in the heart for life and basically forms a part of the heart membrane. Thus there are no real long term concerns related to the device per se.
